
You may be wondering whether CBD is legal to use as a dietary supplement. Good news! The Food and Drug Administration (FDA), has recently removed hemp from its Schedule I drug classification. Although hemp does not contain THC but it is still illegal to include it in food. The FDA regulates cannabis products. In the past CBD and THC products could not be used in food.
Delta-8 THC
The FDA recently issued five warning letters to CBD companies for marketing products with delta-8 THC. These warning letters are the FDA's very first warning regarding delta-8 THC. The letters cite violations of the FDCA, drug misbranding, and the fact that delta-8 THC is not a recognized food additive. No matter if these products prove to be harmful, the FDA will protect Americans' safety.
The FDA is also concerned about products containing delta-8THC, a chemical found naturally in hemp. These products are readily available on the market and can often be marketed as treating medical conditions or diseases. The FDA has sent warning letters out to many companies for marketing products that contain this ingredient. They have a maximum of 15 working days in which to reply. Delta-8 THC products can be dangerous for consumers if the warning letters are true.
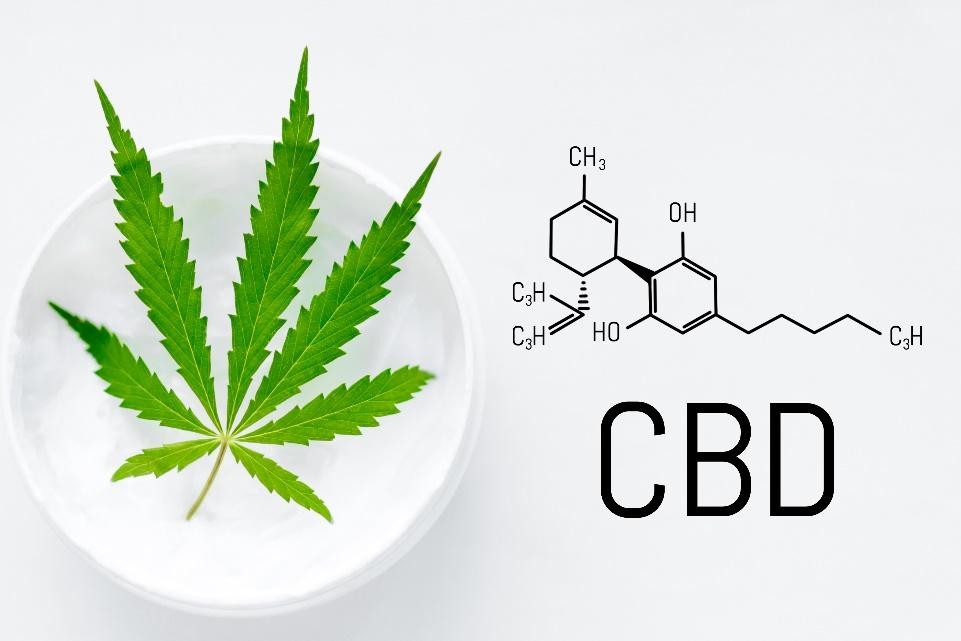
Cannabidiol
FDA sent warning letters to several companies that sell products containing cannabis. CV Sciences, formerly CannaVest was sent two warning letters. Executives from the company responded to the news and stated that they strongly condemned any distributor making misleading or false claims about CBD. This is an important development as it could harm the public's health. FDA must ensure that companies are monitored closely in order to protect public safety and prevent spread of the disease.
FDA has approved Epidiolex as a drug formulation of cannabidiol for treating seizures due to Lennox-Gastaut and Dravet syndromes, and tuberous Sklerose complex. GW Pharmaceuticals expects to get a decision on both indications in the same week. Additionally, the FDA confirmed to the company that it has been granted rare pediatric diseases designation. This could be a precursor of a priority review voucher.
CBD oil
CBD oil is legal and FDA-regulated. The Food and Drug Administration approved the product as legal in the United States. Despite some negative press, CBD oil has been gaining popularity among consumers. Although FDA approval is a huge plus, there's still much to learn. These are important things to remember before you start using CBD oil.
First, make sure you find a company that uses a single process to produce the oil. This will ensure that you are assured of high quality ingredients. The FDA has strict regulations that make it difficult to fake. Finally, it is important to find a independent third-party lab that specializes CBD. Some companies use a single standard process. Make sure to read the labels and look for the word "cannabidiol" in the label.

Cannabidiol is a treatment for epilepsy
Efficacy and safety of cannabidiol as an add-on to standard treatment in patients with drug-resistant epilepsy were found to be improved in a recent open-label study. Three patients with SYNGAP1 epilepsy experienced a reduction in seizures. Bellon M., author the Australian Epilepsy Longitative Survey said that the add on improved the condition of refractory epilepsy sufferers.
These are just a few of the potential drug interactions that were identified by UAB CBD Program. Higher levels of cannabidiol in plasma were associated with better seizure response and improved cognitive function. Researchers also found no evidence of drug interactions with cannabidiol. Therefore, the results of this study provide preliminary support for further research. To determine how CBD impacts these outcomes, more research is required.
FAQ
Which countries have the best quality CBD?
The United States produces most CBD products.
High-quality CBD products are also being produced in Canada, Australia and New Zealand.
What is the size of the global CBD market?
Euromonitor International estimated that the global CBD industry was worth $US3.5 billion in 2015. This is more than 10% higher than 2014
This figure is expected to grow at an average rate of 12% by 2020.
By 2020, CBD products will account for approximately half of all global hemp-derived products.
This includes CBD oils, as well other CBD products, including food, beverages cosmetics, pet care, and CBD oils.
Is the CBD industry growing?
The answer is yes! And that growth is expected not to stop as legalization continues across North America. This year alone, Canada legalized recreational cannabis use, while several states have passed medical marijuana laws.
As more states adopt legislation that allows medicinal marijuana access, this trend is likely to continue at least for the next decade.
Economically, legalizing marijuana makes economic sense. Legalizing pot offers many benefits beyond providing a lucrative market alternative for farmers.
It could be used to reduce crime rates and the availability illegal drugs. It could also provide a source of tax revenue for governments.
People may choose to drink less alcohol as legal marijuana becomes more popular. This would reduce the likelihood of having hangovers. It also means lower healthcare costs.
For chronic pain patients, marijuana may even improve quality of their lives. Many believe that THC (the active ingredient in marijuana) helps to relieve the symptoms of nausea and muscle spasms associated with chemotherapy.
The use of marijuana may be a useful tool in treating mental illness such as anxiety and depression. In fact, some studies suggest that marijuana can even treat schizophrenia.
Even though the CBD sector looks bright, there are still many challenges.
Is CBD's market saturated?
CBD is growing at a rate of more than 25% per year. This growth is expected continue for at most five more years. In fact, the industry is projected to grow from $2 billion today to $5 billion by 2020.
Two companies dominate the CBD market: GW Pharmaceuticals (Canndoc Ltd) and Canndoc Ltd. Both companies have a focus on creating pharmaceutical-grade products. However, they have not been very successful thus far. Both are struggling to get traction on market.
Cannabidiol, or CBD (cannabidiol) is a cannabis extract that contains less 0.3% THC. It has no psychoactive effects. It is used for treating epilepsy and other medical conditions. It is also used frequently as a dietary addition.
There are many varieties of CBD products. Some CBD products contain whole plant extracts. Others use CBD-rich cannabinoids.
These products share one common feature: they all contain low levels of THC.
These products are legal under US federal law. You still need to comply with local laws when you sell CBD products. It is important to check the regulations in your state for CBD products.
Additionally, CBD products can be illegal in several states. These are California, Colorado. Florida. Mississippi. Missouri. New York. North Carolina. Ohio. Oklahoma. Oregon. Rhode Island. South Dakota. Texas. Utah. Virginia. Washington.
CBD products are not recommended for people who live in these states.
How can CBD products be successfully promoted by companies in a regulatory-compliant way?
The FDA does NOT regulate hemp as an agriculture commodity. The Controlled Substances Act regulates other cannabis derivatives (e.g. pot). CBD has not been subject to any specific regulations.
CBD is legal at the state level in 29 states, but federal law still considers it illegal. Businesses looking to sell CBD products are left in uncertainty.
The FDA also maintains strict guidelines on how CBD products may be marketed. For example, they must clearly disclose any product's THC content. Companies cannot claim CBD is effective in treating certain medical conditions without supporting evidence.
Additionally, the FDA requires manufacturers submit information about manufacturing practices and quality control. They also require companies to conduct clinical trials to prove safety and efficacy.
These are important considerations for companies when creating their marketing strategies.
Statistics
- CBD seems unlikely to directly influence sleep in healthy humans [115] (and maybe “sleep-promoting” in those with certain comorbid conditions) (ncbi.nlm.nih.gov)
- A recent study [161] also found that in vitro CBD treatment (i.e., ≤ 2 h exposure to 10 μM) induced ~40% vasorelaxation in isolated (pre-constricted) (ncbi.nlm.nih.gov)
- The use of these products is likely to become even more widespread if the World Health Organization's recommendation that CBD no longer is scheduled in the international drug control conventions is adopted by the United Nations member states [201]. (ncbi.nlm.nih.gov)
- OralWhere HED is the human equivalent dose, and Km is a correction factor estimated by dividing the average body mass (BM) of the species (60, 0.020, and 0.150 kg for 11 humans, mice, and rats, respectively) and by its surface area (see: Nair et al. (ncbi.nlm.nih.gov)
- While the primary injury may not be treatable, interventions that attenuate secondary sequelae are likely to be of benefit [203].Only one study (ncbi.nlm.nih.gov)
External Links
How To
What are the common issues in the CBD industry?
The market for CBD products is expanding at an astounding rate. However, there are still many challenges facing businesses looking to enter this space. These include a lack of consumer awareness, high cost of entry, limited access to capital, and regulatory uncertainty.
Many consumers aren't aware of the benefits and limitations of CBD. This makes it difficult for consumers to make informed decisions on whether or not they want CBD products.
Most CBD companies rely heavily upon word-of mouth marketing. This is costly because they have to pay for advertising and hire staff to promote their brand.
The high production costs are another issue that new entrants to the CBD industry face. High prices are a major problem for CBD products because of the high cost of raw materials. CBD oil can only then be produced if the hemp has been grown in a specific environment.
To grow enough hemp for CBD oil production, it costs approximately $1,000 per acre. This means that many small farmers cannot afford the cost of starting.
Another challenge new entrants face in the CBD market is the lack of access to capital. Due to the stigma surrounding the industry, banks discourage many people who wish to start businesses.
The sale of CBD products is still subject to regulatory uncertainty. There are currently no guidelines on how CBD products should marketed.
While some states have passed legislation restricting CBD products' sale, it has not been adopted as a national policy.
So far, only two states - Maine and Nevada - have legalized recreational marijuana.
Massachusetts and Michigan have considered similar measures.
These changes could mean that CBD manufacturers will be more competitive.
These factors have led many entrepreneurs to choose to work remotely rather than starting a physical business.